Since
the primary chemical reactions in cooking are triggered by heat, let’s take a look at a
chart of the temperatures at which the reactions we’ve just described begin to occur, along
with the temperatures that we commonly use for applying heat to food:
Temperatures of common reactions in food (top portion) and heat sources
(bottom portion).
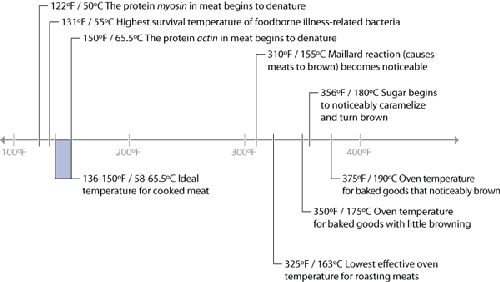
There are a few “big picture” things to notice about these common temperatures in
cooking. For one, notice that browning reactions (Maillard reactions and caramelization)
occur well above the boiling point of water. If you’re cooking something by boiling it in a
pot of water or stewing it in liquid, it’s impossible for high-heat reactions to occur,
because the temperature can’t go much above 216°F / 102°C, the boiling point of moderately
salted water. If you’re cooking a stew, sear the meats
and caramelize the onions separately before adding them to the stew. This way, you’ll get
the rich, complex flavors generated by these browning reactions into the dish. If you were
to stew just the uncooked items, you’d never get these high-heat reactions.
Another neat thing to notice in the temperature graph is the fact that proteins denature
in relatively narrow temperature ranges. When we cook, we’re adding heat to the food
specifically to trigger these chemical and physical reactions. It’s not so much about the
temperature of the oven, grill, or whatever environment you’re cooking in, but the
temperature of the item of food itself.
Which brings us to
our first major aha! moment: the most important variable in cooking is
the temperature of the food itself, not the temperature of the environment in which it’s
being cooked. When grilling a steak, the temperature of the grill will determine how long it
takes the steak to come up to temperature, but at the end of the day, what you really want
to control is the final temperature of the steak, to trigger the needed chemical reactions.
For that steak to be cooked to at least medium rare, you need to heat the meat such that the
meat itself is at a temperature of around 135°F / 57°C.
What’s all this talk about “denaturing” proteins? It’s all about structure. Denaturing refers to a change in the shape of a molecule
(molecular conformation). Proteins are built of a large number of
amino acids linked together and “pushed” into a certain shape upon creation. Since the
function of a protein is related to its shape, changing the shape changes the protein’s
ability to function, usually rendering it useless to the organism. Think of a protein as a bit like the power cable between a laptop and an outlet: while
it has a particular primary structure (the cord and wires inside it), the cord itself
invariably gets all tangled up and twisted into some secondary structure. (If it’s
anything like mine, it spontaneously “retangles” itself regardless of attempts to
straighten it out, but proteins don’t actually do this.) On the molecular level, the cable is the protein structure, and the tangles in the
cable are secondary bonds between various atoms in the structure. Atoms can be relocated
to different bonding spots, changing the overall shape of the molecule, but not actually
changing the chemical composition. With its new shape, however, the molecule isn’t always
able to perform its original function. It might no longer fit into places that it used to
be able to go, or given the new conformation, other molecules might be able to form new
bonds with the molecule and prevent it from functioning as it used to. 
|
1. Heat Transfer and Doneness
The idea that you can just cook a steak any old way until
it reaches 135°F / 57°C sounds too easy, so surely there must be a catch. There are a
few.
For one, how you get the heat into a piece of food matters. A lot. Clearly the center
of the steak will hit 135°F / 57°C faster when placed on a 650°F / 343°C grill than in a
375°F / 190°C oven. The hotter the environment, the faster the mass will heat up, thus the
rule of thumb: “cooking = time * temperature.” Consider the internal temperatures of steak
cooked two ways, grilled and oven-roasted:
Schematic diagram of temperature curves for two imaginary steaks, one
placed in an oven and a second placed on a grill.
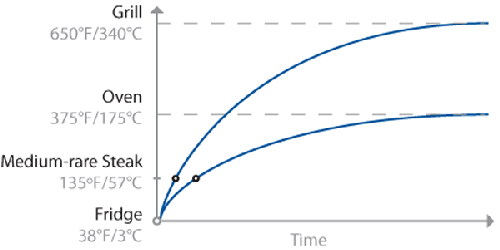
Cooking a steak on a grill takes less time than in an oven, because energy is
transferred faster in the hotter environment of the grill. Note that the error tolerance
of when to pull the meat off the grill is smaller than pulling the meat from the oven,
because the slope of the curve is steeper. That is, if
t1 is the ideal time at which to pull
the steak, leaving it for t1+2 minutes
will allow the temperature of the grilled steak to overshoot much more than one cooked in
the oven.
This is an oversimplification, of course: the graph shows only the temperature at the
center of the mass, leaving out the “slight” detail of the temperature of the rest of the
meat. (It also doesn’t consider things like rate of heat transfer inside the food, water
in the meat boiling off, or points where proteins in the meat undergo phase changes and
absorb energy without a change in temperature.)
Another thing to realize about heat transfer is that it’s not linear. Cooking at a
higher temperature is not like stepping on the pedal to get to the
office faster, where going twice as fast will get you there in half the time. Sure, a
hotter cooking environment like a grill will heat up the outer portions of the steak
faster than a relatively cooler environment like an oven. But the hotter environment will
continue to heat the outer portions of the steak before the center is done, resulting in
an overcooked outer portion compared to the same size steak cooked in an oven to the same
level of internal doneness.
What’s the appeal of cooking on a
hot grill, then? For the right cut of meat, you can keep a larger portion of the center
below the point at which proteins become tough and dry (around 170°F / 77°C) while getting
the outer portion up above 310°F / 154°C, allowing for large amounts of Maillard reactions
to occur. That is, the grill helps give the outside of the steak a nice brown color and
all the wonderful smells that are the hallmark of grilling—aromas that are the result of
Maillard reactions. The outside portion of grilled meat will also have more byproducts
from the Maillard reactions, resulting in a richer flavor.
Juggling time and temperature is a balancing act between achieving some reactions in
some portions of the meat and other reactions in other parts of the meat. If you’re like
me, your ideal piece of red meat is cooked so that the outer crust is over 310°F / 155°C
and the rest of the meat is just over 135°F / 57°C, with as little of the meat between the
crust and the center as possible being above 135°F / 57°C.
This has to be one of the hand-waviest formulas ever. I hereby apologize. To make up
for it, here’s an actual mathematical model for temperature change as a function of heat
being applied. Remember to cook until medium-rare... 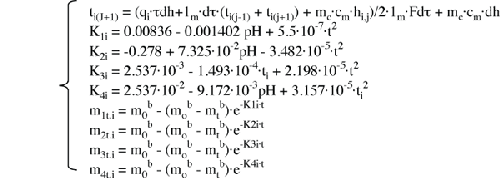
SOURCE: M. A. BELYAEVA (2003), “CHANGE OF MEAT PROTEINS DURING THERMAL TREATMENT,”
CHEMISTRY OF NATURAL COMPOUNDS 39 (4) |
1.1. Temperature gradients
This balancing act—getting the center cooked while not overcooking the outside—has
to do with the rate at which heat energy is transferred to the core of a food. Since
cooking applies heat to foods from the outside in, the outer portions will warm up
faster, and because we want to make sure the entire food is at least above a minimum
temperature, the outside will technically be overcooked by the time the center gets
there. This difference in temperature from the center to outer edges of the food is
referred to as a temperature gradient.
Note:
Choose the method of cooking to match the properties of the food you are cooking.
Smaller items—skirt steak, fish fillets, hamburgers—work well at high heats. Larger
items—roasts, whole birds, meatloaf—do better at moderate temperatures.
Lower heat sources bring up the temperature of the meat more uniformly
than hotter heat sources.
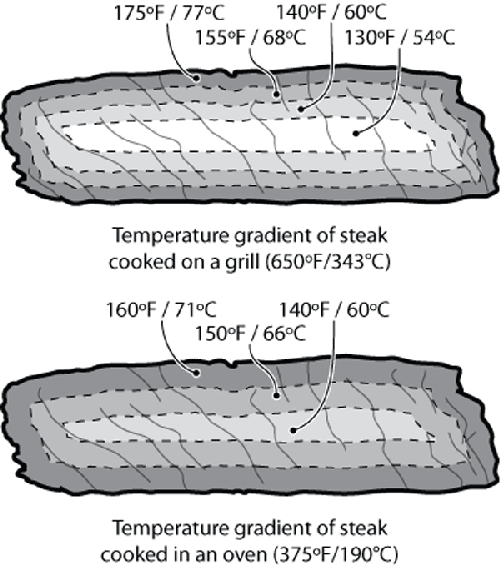
All parts of our example steak are not going
to to reach temperature simultaneously. Because grill environments are hotter than
ovens, the temperature delta between the environment and the food is larger, so foods
cooked on the grill will heat up more quickly and have a steeper temperature
gradient.
1.2. Carryover
Carryover in cooking refers to the phenomenon of continued
cooking once the food is removed from the source of heat. While this seems to violate a
whole bunch of laws of thermodynamics, it’s actually straightforward: the outer portion
of the just-cooked food is hotter than the center portion, so the outer portion will
transfer some of its heat into the center. You can think of it like pouring hot fudge
sauce on top of ice cream: even though there’s no external heat being added to the
system, the ice cream melts because the hot fudge raises its temperature.
The amount of carryover depends upon the mass of the food and the heat gradient, but
as a general rule, I find carryover for small grilled items is often about 5°F / 3°C.
When grilling a steak or other “whole muscle” meat, pull it when it registers a few
degrees lower at its core than your target temperature and let it rest for a few minutes
for the heat to equalize.
Note:
To see how this works, try using a kitchen probe thermometer to record the
temperature of a steak after removing it from the grill once it reaches 140°F / 60°C,
recording data at 30-second intervals. You should see the core temperature peak at
around 145°F / 63°C three minutes into the rest period for a small steak.
Get a cast iron pan good and hot over medium-high heat. Take a steak that’s about
1″ / 2.5 cm thick, rub lightly with olive oil, and sprinkle with salt and pepper. Drop
the steak onto the cast iron pan and let it cook for two minutes. (Don’t poke it! Just
let it sit and sear.) After two minutes, flip and let cook for another two minutes.
Flip again, reduce heat to medium and cook for five to seven minutes, until the center
is about 135°F / 57°C. Let rest on cutting board for five minutes before
serving.