2. Methods of Heat Transfer
There are three methods of transferring heat into
foods: conduction, convection, and radiation. While the heating method doesn’t change the
temperature at which chemical reactions occur, the rate of heat transfer is different
among them, meaning that the length of time needed to cook identical steaks via each
method will be different. The table below shows the common cooking techniques broken out
by their primary means of heat transfer.
2.1. Conduction
Conduction is the simplest type of heat transfer to understand because it’s the most
common: it’s what you experience whenever you touch a cold countertop or grasp a warm
cup of coffee. In cooking, those methods that transfer heat by direct contact between
food and a hot material, such as the hot metal of a skillet, are conduction methods.
Dropping a steak onto a hot cast iron pan, for example, causes thermal energy from the
skillet to be transferred to the colder steak as the neighboring molecules distribute
kinetic energy in an effort to equalize the difference in temperature.
| |
Conduction
|
Convection
|
Radiation
|
|---|
|
Description
|
Heat passes by direct contact between two materials
|
Heat passes via movement of a heated material against a colder
material
|
Heat is transferred via electromagnetic radiation
|
|
Example
|
Steak touching pan; pan touching electric burner
|
Hot water, hot air, or oil moving along outside of food
|
Infrared radiation from charcoal
|
|
Uses
|
Sautéing
Searing
|
Dry heat methods: Baking/roasting, deep-fat
frying
Wet heat methods: Boiling, braising/water bath,
pressure cooking, simmering/poaching, steaming
|
Microwaving
Broiling
Grilling
|
Cooking methods listed by type of heat transfer. (Frying is a dry-heat
method because it does not involve moisture.)
2.2. Convection
Convection methods of heat transfer—baking, roasting,
boiling, steaming—all work by circulating a hot material against a cold one, causing the
two materials to undergo conduction to transfer heat. In baking and roasting, the hot
air of the oven imparts the heat; in boiling and steaming, it’s the water that does
this.
Those heat methods that involve water are called wet heat methods; all others fall
into the dry heat category. One major difference between these two categories is that
wet methods don’t reach the temperatures necessary for Maillard reactions or
caramelization (with the exception of pressure cooking, which does get up to temperature
while remaining moist). The flavorful compounds produced by Maillard reactions in
grilled or oven-roasted items won’t be present in braised or stewed foods: steamed
carrots, for example, won’t undergo any caramelization, leaving the food with a subtler
flavor. Brussels sprouts are commonly boiled and widely hated. Next time you cook them,
quarter them, coat with olive oil and sprinkle with salt, and cook them under a broiler
set to medium.
Note:
Water is an essential material in cooking, and not just for its heat transfer
properties. Rice cookers work by noticing when the temperature rises above 212°F /
100°C. At that point, there’s no water left, so they shut off.
Another key difference between most of the dry versus wet methods is the higher
speed of heat transfer typical in wet methods. Water conducts heat roughly 23 times
faster than air (air’s coefficient of thermal conductivity is 0.026, olive oil’s is
0.17, and water’s is 0.61), which is why hard-cooked eggs finish faster in a wet
environment even at a lower temperature.
Note:
Try it! Cook one egg for 30 minutes in a 325°F / 165°C oven and another for 10
minutes in a 212°F / 100°C water bath. You need to leave the egg in the oven for 20
minutes longer to get the same results.
One exception to this wet-is-faster-than-dry rule is deep-fat frying. Oil is
technically dry (there’s no moisture present), but for culinary purposes it acts a lot
like water: it has a high rate of heat transfer with the added benefit of being hot
enough to trigger a large number of caramelization and Maillard reactions. (Mmm,
donuts!)
Wet methods have their drawbacks (including, depending on the desired result, the
lack of the aforementioned chemical reactions). While the subtler flavors achieved
without browning reactions can be desirable, as in a gently cooked piece of fish, it’s
also much easier to overcook foods with wet methods. When cooking meat, the hot liquid
interacting with it can
quickly raise its temperature above 160°F / 71°C, the point at which a significant
percentage of the actin proteins in meats are denatured, giving the meat a tough, dry
texture. For pieces of meat with large amounts of fat and collagen (such as ribs,
shanks, or poultry legs), this isn’t as much of an issue, because the fats and collagen
(which converts to gelatin) will mask the toughness brought about by the denatured
actin. But for leaner cuts of meat, especially fish and poultry, take care that the meat
doesn’t get too hot! The trick for these low-collagen types of meats is to keep your
liquids at a gentle simmer, around 160°F / 71°C, and minimize the time that the meat
spends in the liquid.
Note:
Even water in its gaseous form—steam—can pack a real thermal punch. While it
doesn’t conduct heat anywhere nearly as quickly as water in its liquid form, steam
gives off a large amount of heat due to the phase transition from gas to liquid,
something that air at the same temperature doesn’t do. As the steam comes into contact
with colder food, it condenses, giving off 540 calories (not to be confused with “food
calories,” which are technically kilocalories) of energy per gram of water, causing
the food to heat up that much more quickly (1 calorie raises the temperature of 1 gram
of water by 1°C).
Steamed vegetables, for example, cook quickly not just because they’re in a 212°F
/ 100°C environment, but also because the water vapor condensing on the food’s surface
imparts a lot of energy. Cheetos, like most “extruded brittle foams” we eat, gain
their puff by being expelled under pressure and heat, which causes them to “steam
puff.” (Think of it as the industrial version of popping popcorn.) There’s a lot of
energy in steam. For this reason, when pouring boiling water through a colander over a
sink, you should be sure to pour away from yourself so that the steam cloud (and any
splashed liquid) doesn’t condense on your face!
2.3. Radiation
Radiant methods of heat transfer impart energy in the form of electromagnetic
energy, typically microwaves or infrared radiation. The warmth you feel when sunlight
hits your skin is radiant heat.
You can create a “heat shield” out of aluminum foil if part of a dish
begins to burn while broiling. The aluminum foil will reflect the thermal
radiation.

In cooking, radiant heat methods are the only ones in which the energy being applied
to the food can be either reflected or absorbed by the food. You can use this reflective
property to redirect energy away from parts of something you’re cooking. One technique
for baking pie shells, for example, includes putting foil around the edge, to prevent
overcooking the outer ring of crust. Likewise, if you’re broiling something, such as a
chicken, and part of the meat is starting to burn, you can put a small piece of
aluminum foil directly on top of that part of meat.
2.4. Combinations of heat
The various techniques for applying heat to food differ in other ways than just the
mechanisms of heat transfer. In roasting and baking we apply heat from all directions,
while in searing and sautéing heat is applied from only one side. This is why we flip
pancakes (stovetop, heat from below) but not cakes (oven, heat from all directions). The
same food can turn out vastly different under different heat conditions. Batter for
pancakes (conduction via stovetop) is similar to that for muffins (convection via
baking) and waffles (conduction), but the end result differs widely.
To further complicate things, most cooking methods are actually combinations of
different types of heat transfer. Broiling, for example, primarily heats the food via
thermal radiation, but the surrounding air in the oven also heats up as it comes in
contact with the oven walls, then comes in contact with the food and supplies additional
heat via convection. Likewise, baking is primarily convection (via hot air) but also
some amount of radiation (from the hot oven walls). “Convection ovens” are nothing more
than normal ovens with a blower inside to help move the air around more quickly. All
ovens are, by definition, convection ovens, in the sense that heat is transferred by the
movement of hot air. Adding a fan just moves the air more quickly, leading to a higher
temperature difference at the surface of the (cold) food you’re cooking.
To a kitchen newbie, working with combinations of heat might be frustrating, but as
you get experience with different heat sources and come to understand how they differ,
you’ll be able to switch methods in the middle of cooking to adjust how a food item is
heating up. For example, if you like your lasagna like I do—toasty warm in the middle
and with a delicious browned top—the middle needs to get hot enough to melt the cheese
and allow the flavors to meld, while the top needs to be hot enough to brown. Baking
alone won’t generate much of a toasted top, and broiling won’t produce a warm center.
However, baking until it’s almost done and then switching to the broiler achieves both
results.
Note:
The convenience food industry cooks with combinations of heat, too, cooking some
foods in a hot oven while simultaneously hitting them with microwaves and infrared
radiation to cook them quickly.
When cooking, if something isn’t coming out as you
expect—too hot in one part, too cold in another—check to see whether switching to a
different cooking technique can get you the results you want.
If you’re an experienced cook, try changing heat sources as a way of creating a
challenge for yourself: adapt a recipe to use a different source of heat. In some cases,
the adaptation is already common—pancake batter, when deep-fat fried, is a lot like
funnel cakes. But try pushing things further. Eggs cooked on top of rice in a rice
cooker? Chocolate cookies cooked in a waffle iron? Fish cooked in a dishwasher? Why not?
It might be unconventional, but heat is heat. Sure, different sources of heat
transfer energy at different rates, and some are better suited to transitioning the
starting thermal gradient (edge to center) of the food to the target thermal gradient.
But there are invariably similar enough heat sources worth trying. And you can push it
pretty far: fry an egg on your CPU, or cook your beans and sausage on an engine block
like some long-haul truck drivers do! As a way of getting unstuck—or just playing
around—it’s fun to try.
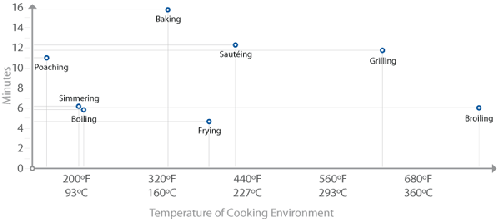
Cooking methods plotted by rate of heat transfer. This plot shows the
amount of time it took to heat the center of uniformly sized pieces of tofu from
40°F / 4°C to 140°F / 60°C for each cooking method. Pan material (cast iron,
stainless steel, aluminum) and baking pan material (glass, ceramic) had only minor
impact on total time for this experiment and are not individually
listed.