While yeast
allows for the creation of many delicious foods, it has two potential drawbacks: time and
flavor. Commercial bakers with high volumes and those of us with limited time to play in the
kitchen can’t always afford to wait for yeast to do its thing. Then there’re the flavors and
aromas generated by yeast, which would clash with the flavors in something like a chocolate
cake. Chemical leaveners have neither of these problems.Chemical leaveners are divided into two categories:
Baking soda
A bicarbonate (HCO3–) that’s bound
with another molecule—typically sodium, but sometimes potassium and ammonium. When added
to water, the bicarbonate dissolves and is able to react with acids to generate
CO2.
Baking powder
A self-contained leavening system that generates carbon dioxide in the presence of
water. Baking powders by definition contain a baking soda and acids for that baking soda
to react with.
The idea that these are categories, not single ingredients, is probably foreign to most
home cooks, but the chemicals that make up a baking powder or baking soda can vary.
Industrial food manufacturers use different compositions and particulate sizes depending
upon the food being produced.
1. Baking Soda
Anyone who’s done the third-grade science fair project using vinegar and baking soda
to make a volcano can tell you that baking soda can generate a whole lot of gas really
quickly. But in the kitchen, baking soda remains one of the bigger mysteries. How is it
different from baking powder? And how do you know which one to use?
The quick answer would go something like: “Baking soda reacts with acid, so only use
it when your ingredients are acidic.” And as simple explanations go, this covers you 99%
of the time when cooking. But baking soda is a little more complicated and interesting in
a geeky way, so it’s worth a brief digression into the chemistry. I promise this’ll be
short.
The baking soda you buy in the store is a specific chemical: sodium bicarbonate,
NaHCO3. Unlike baking powder, which is a blend of chemicals that
are self-contained (“just add water and heat!”), when added to a dish, sodium bicarbonate
needs something to react with in order to generate gas.
Always sift dry ingredients together before adding in wet ingredients to
make sure any salt, baking soda, or baking powder are truly dispersed. You can use a
strainer over a bowl as a sifter or even just mix the ingredients with a wire whisk
or a fork.

Without
something for sodium bicarbonate to dissolve into, it’s an inert white powder. Upon
getting wet—any moisture in any food will do—the sodium bicarbonate dissolves, meaning
that the sodium ions are free to run around separately from the bicarbonate ions.
Note:
The sodium is just there to transport the bicarbonate to your food; we can ignore it
once it’s dissolved. The sodium does make the food slightly saltier, incidentally, which
is why industrial food manufacturers will sometimes use things like potassium
bicarbonate: potassium is good for you, and this avoids the sodium for people on a
low-sodium diet.
Most of us are familiar with the pH scale (the H stands for hydrogen; it’s unclear
what the p stands for, “power” and “potential” are the best guesses). The pH scale is a
measure of the amount of available hydrogen ions in a solution. Chemicals that affect the
number of hydrogen ions can be classified in one of two ways:
Acids (pH below 7)
Proton donors; i.e., chemicals that increase the number of hydronium ions
(H3O+; the hydrogen binds with a
water molecule) in the solution
Bases (pH above 7)
Proton receivers; i.e., chemicals that bind with hydronium ions, reducing their
available concentration in a solution
When it comes to pH, a bicarbonate ion has an interesting property that chemists call
amphotericity: it can react with either an acid or a base. In the
kitchen, so few things are actually basic—egg whites, baking soda, maybe the stuff in your
fire extinguisher, and that’s pretty much it—that you can safely ignore baking soda’s
ability to react with bases and just think of it as something that reacts with acids.
Still, to understand baking soda, it’s important to understand that bicarbonates react
with other compounds and either raise the pH by reducing the amount of available acids or
lower the pH by reducing the amount of available bases.
This phenomenon is called buffering: a
buffer is something that stabilizes the pH level of a solution.
Buffers hang out in the solution and, when an acid or base is added, glom on to it and
prevent it from affecting the count of available hydronium ions. In a glass of pure water,
there’s not much for the bicarbonate ions from baking soda to interact with, so they just
float around and taste generally nasty. But if you were to add a spoonful of vinegar—which
is acetic acid—to that glass, the bicarbonate ions would react with the acetic acid and
generate carbon dioxide as part of that reaction.
Depending upon the amount of bicarbonate you
started with, after you add the spoonful of vinegar the glass will be in one of three
states (none of which involve being half-full or half-empty): bicarbonate ions still
available but no acetic acid ions available, no bicarbonate ions available but acetic acid
ions still available, or neither bicarbonate nor acetic acid ions freely available. In
baking, it’s this last state—a neutral balance—that we want to reach. Too much baking
soda, and it won’t all react with the acids in the food and will leave the food with a
soapy, yucky taste. Not enough baking soda, and the food will remain slightly acidic
(which is okay) and not have as much lift as possible (which is probably not okay—your
food will be flat). To repeat one of my favorite quotes: “Dosage matters!”
The reaction between baking soda and an acid is the key to understanding when you
should use baking soda versus baking powder. This balancing act between acids and baking
soda isn’t a problem with baking powder, of course. This is because the baking powder is
already balanced for you—the ratio of acids to bicarbonate is preset by the
manufacturer.
If your ingredients aren’t very acidic, baking soda won’t have much to react with, so
use baking powder. On the other hand, if your ingredients are extremely acidic, using
baking soda will work, since there will be enough hydronium ions to react with. How much
baking soda to use depends on the pH of the ingredients in your dish. Short of testing or
calculating the pH, experimentation is the easiest way: take a guess and keep notes. Keep
adding baking soda until the additional baking soda no longer helps with lift (or can be
tasted). If you’re still not getting enough lift at this point, switch to adding baking
powder.
Note:
Baking soda doesn’t need an acid to decompose; heat will do it, too. Try melting
some sugar, just as though you were making caramel , and instead of adding
cream, add a small spoonful of baking soda and stir. The baking soda will break down and
cause the sugar to bubble up.
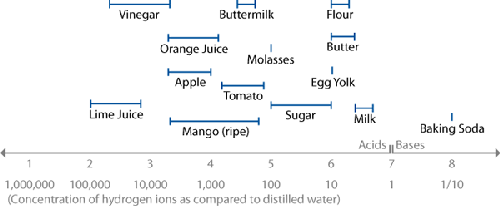
The pH of common ingredients.
Given time, yeast and bacteria
generate flavors that we often find pleasant. But what about those times when you’re
craving that taste right now—or at least, sometime this morning? You can take a
shortcut by using buttermilk, which has already been munched on by
bacteria.
Whisk together to combine thoroughly:
2 cups (240g) bread flour
5 tablespoons (60g) sugar
1 ½ teaspoons (7g) baking soda
1 teaspoon (5g) salt
In a separate bowl, melt:
½ cup (115g) melted butter
In the same bowl as the butter, add and whisk together:
2 ½ cups (610g) buttermilk (lukewarm!)
2 large (120g) eggs
Mix the wet ingredients into the dry, stirring with a whisk or spoon to combine.
Cook on a griddle or nonstick frying pan set over medium heat (if you have an IR
thermometer, 325–350°F / 160–175°C) until golden brown, about two minutes per
side.
Notes
You don’t need to butter the griddle or pan before cooking these—there
is enough butter in the batter that the pancakes are self-lubricating—but if you
do feel the need, wipe any excess butter out of the pan before cooking the
pancakes. If you have any dots of oil on the surface, they’ll interfere with the
Maillard browning reactions.
Pull the buttermilk and eggs out of the fridge an hour or so before
you’re ready to use them, to allow them to come up to room temperature. If you’re
in a rush, you can double-duty a microwave-safe mixing bowl: melt the butter in
it, add the buttermilk, then nuke it for 30 seconds to raise the temperature of
the buttermilk.
Note:
Try using this batter for buttermilk fried chicken. Slice cooked chicken into
bite-sized pieces, dredge them in cornstarch, dip them in this batter, and then
deep-fry them in vegetable oil at 375°F / 190°C. The starch will help the batter
adhere to the chicken. (No cornstarch? Use flour.)