2. Colloids
One of the more common uses of industrial chemicals in food is to form colloids. A
colloid is any mixture of two substances—gas, liquid, or solid—where one is uniformly
dispersed in the other, but they are not actually dissolved together. That is, the two
compounds in the mixture don’t form chemical bonds, but the overall structure appears
uniform to the naked eye.
Common colloids in the kitchen are whole milk and chocolate. In milk, solid particles
of fat are dispersed throughout a water-based solution. In chocolate, particles of cocoa
solids are dispersed throughout a solid medium of cocoa fat and other ingredients.
The following table shows the different combinations of particles and media, along
with examples of foods for each colloid type. The medium of a colloid is called the
continuous phase (it’s the watery liquid in milk); the particles
are known as the dispersed phase (for milk, the fat droplets).
| |
Gas particles
|
Liquid particles
|
Solid particles
|
|---|
|
Gas medium
|
(N/A: gas molecules don’t have a collective structure, so gas/gas
combinations either mix to create a solution or separate out due to
gravity)
|
Liquid aerosols
Mist sprays
|
Solid aerosols
Smoke (convertible to a solid-in-liquid colloid via liquid smoke)
Aerosolized chocolate
|
|
Liquid medium
|
Foams
Whipped cream
|
Emulsions
Milk
Mayonnaise
|
Sols and suspensions
Commercial salad dressings
|
|
Solid medium
|
Solid foams
Meringue cookies
Soufflés
|
Gel
Gelatin
Jell-O
|
Solid sols
Chocolate
|
|
Some of these colloid types might remind you of various dishes
served at more experimental restaurants.
|
One of the surprises of this table is the relatively broad swath of techniques that it
captures. Foams, spherifications, and gelled foods are all colloids. Even some of the more
recent novel dishes are colloids from the gas medium category. Chef Grant Achatz (Alinea,
in Chicago) has used solid aerosols by infusing a pillow with smoke and then placing the
dish on top of the pillow, forcing the air containing the aerosol to leave the pillow and
diffuse into the diner’s environment.
Note:
Chef Achatz uses smoke-infused “pillows” to present a pleasant olfactory experience
while avoiding the taste sensation for items such as mace and lavender.
Other luxury restaurants have created courses that involve liquid aerosols (by
spraying a perfume), and one company (Le Whif) is working on a kitchen gadget that creates
solid aerosols from foods such as chocolates.
Some food additives can be used in more than one type of colloid. For example, guar
gum can act as an emulsifier (by preventing droplets of oil from coalescing) and as a
stabilizer (by preventing solids from settling). Methylcellulose is both a gelling agent
and an emulsifier. Don’t think of food additives as directly mapping onto the colloids
they create, but it’s a handy framework for thinking about the types of effects you can
achieve.
3. Making Gels: Starches, Carrageenan, Agar, and Sodium Alginate
The food
industry uses gels to thicken liquids, to emulsify sauces, to modify texture (“improve
mouth-feel,” as they say), and to prevent crystal formation in products such as candies
(sugar crystals) and ice cream (ice crystals and sugar crystals). Gels are also found in
traditional home cooking: both gelatin and pectin are used in many dishes to improve
mouth-feel, and they also help preserve items such as jams.
From the perspective of modernist cuisine, thickeners and gels are used primarily to
create dishes in which foods that are typically liquid are converted into something that
is thick enough to hold its shape (this is what pectin does in jam), or even completely
solid.
Gels can also be formed “around” liquids to create a gelatinous surface in a technique
known as spherification, originally discovered by Unilever in the
1950s and brought to the modernist cuisine movement by Chef Ferran Adrià of elBulli. For
our purposes, gels in foods can be classified into two general types: soft gels and
brittle gels (true gels).
You can think of a soft gel as a thicker version of the original
liquid: it has increased viscosity (it’s “thicker”), but it retains its ability to flow.
Soft gels can exhibit a phenomenon termed shear thinning, which is
when a substance holds its shape but will flow and change shape when pressure is applied.
Substances like ketchup and toothpaste exhibit shear thinning: squeeze the bottle or tube,
and it flows easily, but let go, and it holds its shape.
Iota carrageenan (left, 2% concentration) creates a flexible brittle
gel, while kappa carrageenan (right, 2% concentration) creates a firm brittle gel.
These two samples are resting on top of a narrow bar.
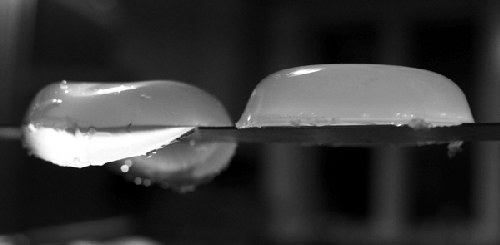
While a soft gel can be described as a “thicker” version of the original liquid, a
brittle gel can be thought of as a solid. Brittle gels—foods like
cooked egg whites and Jell-O—have a tightly interconnected lattice that prevents them from
flowing at all. With sufficient quantities of the gelling agent, this type can form a
block or sheet that you can pick up, slice into blocks or strips, and stack as a component
in a dish, and it has a “memory” of its cast shape, meaning that it will revert to that
shape when no other forces are in play.
In the consumer kitchen, cornstarch is the standard traditional gelling agent. In
industrial cooking, carrageenan is commonly used in gelling applications. (Try finding
cream cheese that doesn’t have carrageenan in it.) Iota carrageenan is used when a
thickening agent is needed, while kappa carrageenan and agar yield firm, brittle gels.
While the gelling agents used to create flexible and rigid gels are generally different,
you can create a flexible gel with a gelling agent typically used in rigid, brittle
applications by carefully controlling the quantity of gelling agent used.
3.1. Making gels: Starches
Starches are used as thickeners in
everything from simple roux to pie filling. They’re easy, plentiful, and exist in almost
all of the world’s cuisines: cornstarch, wheat flour, tapioca starch, and potato “flour”
(not actually a flour) being the most common. While there are differences among these
starches—size of the starch granules, length of the molecular structure, and variations
on the crystalline structure—they all act essentially the same. Expose to water, heat
up, then cool down, and they thicken up.
Gelatinization temperature of common starches.
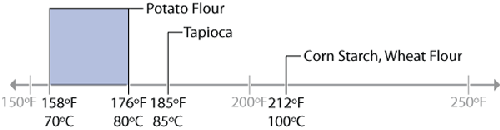
Starch is composed of repeating units of amylopectin and amylose that form
crystalline structures. The gelatinization temperature—the temperature at which these
crystalline structures melt and then absorb water and swell—can vary, depending upon the
ratio of amylopectin and amylose groups. We’ll examine cornstarch here, but as you play
with the others, keep in mind that the gelatinization temperature can vary.
Instructions for use. To use cornstarch (called “corn flour” in the UK) to make a gel, mix it with a
small amount of cold liquid such as water to create a slurry. Adding cornstarch
directly to a hot liquid will result in clumps. Add the slurry to the desired dish
and bring to a simmer.
Uses. Cornstarch is used as a thickener and has about twice the thickening ability
of flour. When a recipe calls for a teaspoon of flour, use half a teaspoon of
cornstarch. Cornstarch is gluten-free, making it a good thickening substitute for
those with gluten allergies.
(Flour isn’t as good a thickener because it contains other stuff in addition to
starch, such as gluten, fat, fiber, and minerals.)
Origin and chemistry. Derived from corn (shocker, I know). Like other starches used in cooking
(e.g., potato, tapioca, wheat), cornstarch is a carbohydrate composed of repeating
units of amylopectin and amylose that form crystalline structures. On heating,
these structures swell up and break down. Upon cooling, the leached amylose
molecules can link together to create a 3D mesh, trapping other molecules into the
network. For more on the chemistry of starches, see http://www1.lsbu.ac.uk/water/hysta.html.
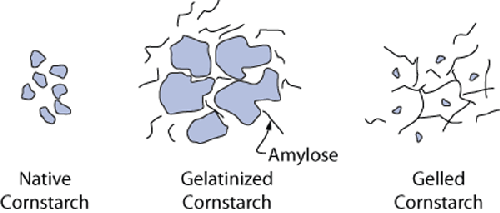
|
Technical notes
|
|---|
|
Gelatinization temperature
|
203°F / 95°C; maximum thickness at 212°F / 100°C.
|
|
Gel type
|
Thixotropic. (This means it becomes less viscous when pressure is applied.
Think ketchup: it holds its shape, but flows under pressure.)
|
|
Syneresis (“weeping”)
|
Extensive if frozen and then thawed.
|
|
Thermoreversible
|
No—after gelatinizing, the amylose is leached out from the original starch
molecules.
|
Like many savory foods in which
multiple discrete components are combined to create the dish, lemon meringue pie is
the combination of three separate components: pie dough, a meringue, and a
custard-like filling. We’ve already covered pie dough and meringues , so the only thing left for
making a lemon meringue pie is the filling itself. Flip to those recipes for
instructions on how to make the pie dough and meringue topping.

To make the lemon custard, place in a saucepan off heat and whisk together:
2
½ cups (500g) sugar
¾ cup (100g) cornstarch
½ teaspoon (5g) salt
Add 3 cups (700g) of water, whisk together, and place over medium heat. Stir until
boiling and the cornstarch has set. Remove from heat.
In a separate bowl, whisk together:
6 egg yolks
Save the whites for making the meringue. Make sure not to get any egg yolk in the
whites! The fats in the yolk (nonpolar) will prevent the whites from being able to
form a foam when whisked.
Slowly add about a quarter of the cornstarch mixture to the egg yolks while
whisking continuously. This will mix the yolks into the cornstarch mixture without
cooking the egg yolks (tempering). Transfer the entire egg mixture back into the
saucepan, whisk in the following ingredients, and return to medium heat and cook until
the eggs are set, about a minute:
1 cup (240g) lemon juice (juice of about 4
lemons)
Zest from the lemons (optional; skip if using bottled
lemon juice)
Transfer the filling to a prebaked pie shell. Cover with Italian meringue made
using the six egg whites , and
bake in a preheated oven at 375°F / 190°C for 10 to 15 minutes, until the meringue
begins to turn brown on top. Remove and let cool for at least four hours—unless you
want to serve it in soup bowls with spoons—so that the cornstarch has time to
gel.
To create decorative peaks on the meringue, use the back of a spoon:
touch the surface of the unbaked meringue and pull upward. The meringue will
stick to the back of the spoon and form peaks.

Note:
Gelling agents typically come as a powdered substance that is added to water or
whatever other liquid you are working with. Upon mixing with the liquid, and
typically after heating, the gelling agent rehydrates and as it cools forms a
three-dimensional lattice that “traps” the rest of the liquid in suspension. By
default, add your gelling agent to a cold liquid and heat that up. Adding gelling
agents to hot liquid usually results in clumps because the outer layer of the powder
will gel up around the rest of the powder.