4. Making Things Melt in Weird Ways: Methylcellulose and Maltodextrin
At a high level, making gels is about
transforming liquids into solids. In addition to creating gels, though, modern food
additives can be used to alter other properties of foods, and another area of play in the
modernist kitchen is that of melting. How can we make things change state in unexpected
ways?
4.1. “Melts” as it cools: Methylcellulose
Methylcellulose has the unusual property of getting thicker when heated
(thermo-gelling in chem-speak). Take jam: when heated, it loses
its gel structure (the pectin melts), causing it to flow out of things like jam-filled
pastries. Adding methylcellulose prevents this by causing the jam to “gel” into a solid
under heat. And since methylcellulose is thermoreversible, upon cooling after baking,
the jam returns to its normal consistency.
Note:
Hollywood uses methylcellulose to make slime. Add a bit of yellow and green food
dye, and you’ve got yourself Ghostbusters-style slime. To get
good consistency, whisk it vigorously to trap air bubbles into the mixture.
Methylcellulose has been used in some modernist cuisine dishes for its
thermo-gelling effects. One famous example is “Hot Ice Cream” in which the “ice” cream
is actually hot cream that’s been set with methylcellulose. As it cools to room
temperature, it melts.
Instructions for use. Dissolve methylcellulose into hot water (above 122°F / 50°C) and then whisk
while cooling down. Mixing it directly in cold water can be difficult because the
powder will clump up as it comes into contact with water. In hot water, though,
the powder doesn’t absorb any water, allowing it to be uniformly mixed. It’s
easiest to stir in 1.0% to 2.0% (by weight) into your liquid and let it rest
overnight in the fridge to dissolve fully. You can then experiment with setting
the liquid. Try baking a small dollop of it, or dropping it by the ice cream
scoopful into a pan of simmering water.
Uses. Commercial applications use it to prevent “bake-out” of fillings in baked
goods. Methylcellulose also has high surface activity, meaning that it acts as an
emulsifier by keeping oil and water from separating, so it is also used in low-oil
and no-oil dressings and to lower oil absorption in fried foods.
Note:
Methylcellulose increases surface tension—well, actually, “interfacial tension”
because “surface” refers to a two-dimensional shape—which is why it works as an
emulsifier.
Origin and chemistry. Methylcellulose is made by chemically modifying cellulose (via etherification
of the hydroxyl groups). There can be great variation between types and
derivatives of methylcellulose, in terms of thickness (viscosity), gelling
temperature (122–194°F / 50–90°C), and strength of gel (ranging from firm to
soft). If you’re having problems getting your methylcellulose to set, check the
specifications of the type you have.
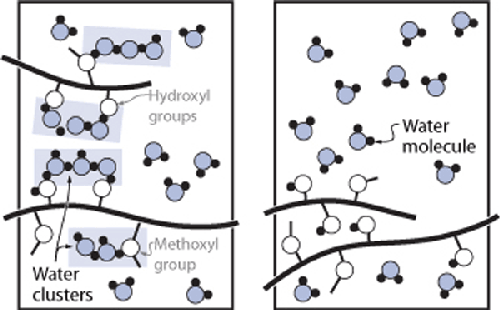
When cold (on left), water molecules are able to form water clusters
around the methylcellulose molecule. With sufficient heat—around 122°F / 50°C—the
water clusters are destroyed and the methylcellulose is able to form crosslinks,
resulting in a stable gel at higher temperatures.
In a saucepan, bring to a boil:
2⅛ cups (500g) water
1 cup (200g) sugar
Let cool, and then whisk in:
10g methylcellulose (use a scale to ensure an accurate
measurement)
1 teaspoon (5g) vanilla extract
Let rest in fridge until thick, around two hours. Once thick, whisk until light
and foamy. Transfer to a 9″ × 9″ / 20 cm × 20 cm baking pan lined with parchment
paper. Bake for five to eight minutes at 300°F / 150°C, until set. The marshmallows
should feel dry to the touch and not at all sticky. Remove from oven, cut into desired
shapes, and coat with powdered sugar.
Two marshmallows on a plate of powdered sugar.

Two marshmallows after being coated with powdered sugar while still
hot.

Same marshmallows after cooling for a few minutes.

When working with gels, you can quickly cool the hot liquid by
whisking it while running cold water over the outside of the pan. The water will
flow along the bottom of the pan.
