6.2.5. Making Foams: Lecithin
Foams are another area of
play in modernist cuisine. If you ever happen to be served a dish that has a “foam”
component—say, cod served on a bed of rice with a “carrot” foam or
uni (sea urchin) in a shell with green apple foam, it was probably
created by adding a stabilizer such as lecithin or methylcellulose to a liquid and then
whipping or puréeing it. While perhaps a little too trendy, it’s a clever way to
introduce a flavor to a dish without adding much body.
Instructions for use. Add about 1% to 2% lecithin to your liquid (by weight, i.e., 1g lecithin per
100g liquid) and use an immersion blender to froth the liquid. Hold the immersion
blender up and at a slight angle so that the blades are in contact with both the
liquid and air.
Uses. As an emulsifier, lecithin can be used to create stable flavored foams. It’s
also used as an antispattering agent in margarines, an emulsifier in chocolate (to
reduce the viscosity of the melted chocolate during manufacturing), and as an active
ingredient in nonstick food sprays.
Origin and chemistry. Lecithin is typically derived from soya beans as a byproduct of creating
soy-based vegetable oil. Lecithin is extracted from hulled, cooked soya beans by
crushing the beans and then mechanically separating out (via extraction, filtration,
and washing) crude lecithin. The crude lecithin is then either enzymatically
modified or extracted with solvents (e.g., de-oiling with acetone or fractionating
via alcohol). Lecithin can also be derived from animal sources, such as eggs and
animal proteins, but animal-derived lecithin is much more expensive than
plant-derived lecithin, so it is less common.
Lecithin molecules have polar and non-polar regions that are most stable
when one side is exposed to a polar substance and the other side to a nonpolar
substance. See the sidebar The Chemistry of Emulsifiers for a
description of how lecithin stabilizes foams.
In a large mixing bowl or other similarly large and flat container, blend with an
immersion blender: ½ cup (100g) water ½ cup (100g) juice, such as carrot, lime, or
cranberry 1 teaspoon (3g) lecithin (powder)
Notes Hold the immersion blender such that it is partly out of the liquid.
You want to allow it to siphon air into the mixture. Allow foam to rest for a minute after blending, so that the resulting
foam that you spoon off is more stable. Try other liquids, such as coffee or beet juice. Lecithin works best
at around a 1–2% concentration (2g lecithin per 100g of liquid).
Lecithin can be used to make a large-bubble foam that is
surprisingly stable for long periods of time.

|
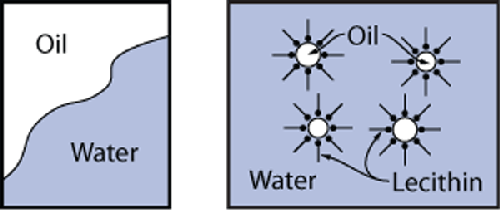
You might be wondering why oil and water are able to “mix”
in the presence of an emulsifying agent, after the earlier discussion about polar (e.g.,
water) versus non-polar (e.g., oil) molecules not being able to mix. An emulsifier has a
hydrophilic/lipophilic structure: part of the molecule is polar and thus “likes” the
water, and part of the molecule is nonpolar and “likes” the oil. Emulsifiers concentrate
at the boundary between water and oil because of the charge structure of the
molecules.
Adding an emulsifier keeps foods from separating by providing a barrier between
droplets of oil. Think of it like a skin around the oil droplets that prevents different
droplets from touching and coalescing. Emulsifiers reduce the chance that oil droplets
will aggregate by increasing what chemists call interfacial
tension. The oil and water don’t actually mix; they’re just held apart at the
microscopic level.
Emulsifiers stabilize foams by increasing their kinetic stability—i.e., the amount
of energy needed to get the foam to transition from one state to another is higher. Take
the foam of a bubble bath as an example: the soap acts as an emulsifier, creating a foam
of air and water. Water doesn’t normally hold on to air bubbles, but with the soap (the
emulsifier), the interfacial tension between the air and water goes way, way up, so it
takes more energy to disrupt the system. The more energy it takes, the more kinetically
stable the foam is, and the longer it’ll last.
A photo under a light microscope of a half-water, half-oil solution.
(The slide is pressing the oil droplets flat.)

The same mixture with 1% lecithin added. The droplets are stable and
do not coalesce into larger drops.
