2. 144°F / 62°C: Eggs Begin to Set
The lore of eggs is
perhaps greater than that of any other food item, and more than one chef has gone on
record judging others based on their ability—or inability—to cook an egg. Eggs are the
wonder food of the kitchen—they have a light part, a dark part, and bind the culinary
world together. Used in both savory and sweet foods, they act as binders holding together
meatloaf and stuffing; as rising agents in soufflés, certain cakes, and cookies like
meringues; and as emulsifiers in sauces like mayonnaise and hollandaise. Eggs provide
structure to custards and body to ice creams. And all of this so far doesn’t even touch on
their flavor or the simple joys of a perfectly cooked farm egg. Simply put, I cannot think
of another ingredient whose absence would bring my cooking to a halt faster than the
simple egg.
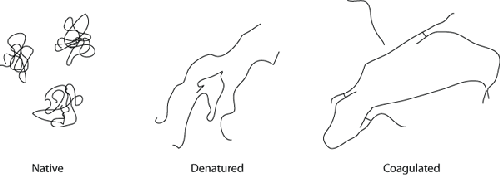
Egg whites are composed of dozens of different types of proteins, and each type of
protein begins to denature at a different temperature. In their natural “native” state,
you can think of the proteins as curled-up little balls. They take this shape because
portions of the molecular structure are hydrophobic—the molecular
arrangement of the atoms making up the protein is such that regions of the protein are
electromagnetically repulsed by the polar charge of water.
Important temperatures in eggs.

Because of this aversion to water, the protein structure folds up on itself. As
kinetic energy is added to the system—in the form of heat or mechanical energy (e.g.,
whipping egg whites)—the structure starts to unfold as kinetic energy overtakes potential
energy. The unfolded proteins then get tangled together, “snagging” around other denatured
proteins and coagulating to form a linked structure. This is why a raw egg white is
liquid, but once cooked becomes solid.
Hydrophobic proteins in their native state
(left) remain curled up to avoid interacting with the surrounding liquid. Under
heat, they denature (center) and uncurl as the kinetic energy exceeds the weaker
level of energy generated by water molecules and regions of the proteins that repel
each other. Once denatured and opened up, the hydrophobic parts of the protein that
were previously unexposed can interact and bond with other
proteins.
The most heat-sensitive protein is ovotransferrin, which begins to denature at around
144°F / 62°C. Another protein, ovalbumin, denatures at around 176°F / 80°C. These two
proteins also are the most common in egg whites: ovotransferrin accounts for 12% of the
proteins in an egg white and ovalbumin 54%. This explains the difference between
soft-boiled and hard-boiled (“hard-cooked”) eggs. Get that egg up to about 176°F / 80°C
for sufficient time, and voilà, the white is hard cooked; below that temperature, however,
the ovalbumin proteins remain curled up, leaving the majority of the egg white in its
“liquid” state.
Note:
Most of the proteins in egg yolks set at between 149°F / 65°C and 158°F / 70°C,
although some set at lower temperatures.
Proteins in foods such as eggs don’t denature instantaneously once they reach
denaturation temperature. This is an important point. Some cooking newbies have the mental
model that cooking an egg or a piece of meat is something like melting an ice cube: all
ice below a certain temperature, ice and water at the freezing/melting point, and all
water above that temperature. From a practical perspective in the kitchen, it’s not an
entirely incorrect picture, because heat pours into the foods so quickly that the subtle
differences between a few degrees aren’t obvious. But as heat is transferred into the food
more slowly, the subtleties of these chemical reactions become more noticeable. And unlike
melting an ice cube, where increasing the heat transfer by a factor of two causes the ice
to melt in half the time, cooking foods do not respond to additional energy in a linear
fashion.
You might find
it easiest to think of the different proteins in foods as having particular temperatures
at which they denature, and try to shoot for a target temperature just above that of the
proteins you do want denatured. Just remember: there’s more to a piece of meat or egg than
one type of protein or connective tissue, and the different proteins have different
temperature points at which they’re likely to denature.
Here are some examples of cooking eggs that show how to take advantage of the thermal
properties of different portions of the egg.
2.1. Hard-Cooked Eggs, Shock and Awe Method
There’s a silent war of PC-versus-Mac proportions going on over the ideal way to
make hard-cooked eggs. Should you start in cold water and bring the water up to a boil
with the eggs in them, or should you drop the eggs into already boiling water? The
cold-start approach yields eggs that taste better, while the boiling-water approach
yields eggs that are easier to peel. But can you have both?
Thinking about the thermal gradient from shell to center of egg, it would make sense
that cooking an egg starting in cold water would result in a more uniform doneness. The
delta between the center and outer temperatures will be smaller, meaning that the outer
portion won’t be as overcooked once the center is set compared to the boiling-water
method.
The conjecture for ease of peeling in the boiling water approach is that the hot
water “shocks” the outer portion of the egg. Into industrial-grade cooking? Steam ’em at
7.5 PSI over atmospheric pressure and quick-release the pressure at the end of cooking
to crack the shell. (Hmm, I wonder if one could do this in a pressure cooker...) But
what about the rest of us? What if we shock the outside, and then cook in cold
water?
Try it. Place your eggs into rapidly boiling water. After 30 seconds, transfer the
eggs to a second pot containing cold tap water, bring to a boil, and then simmer. The
second-stage cooking time will take about two minutes less than the normal cold-start
approach. Cook for 8 to 12 minutes, depending upon how well cooked you like your
eggs.
2.2. The 30-Minute Scrambled Egg
This method involves ultra-low heat, continuous stirring, and a vigilant eye. I
wouldn’t suggest this as an everyday recipe, because it takes a while to make, but after
however many years of eating eggs, it’s nice to have them cooked a new way. Cooking the
eggs over very low heat while continuously stirring breaks up the curds and allows for
cooking the eggs to a point where they’re just cooked, giving them a flavor that can be
described as cheese or cream-like. It’s really amazing, and while the thought of “cheese
or cream-like” eggs might not have you racing off to the kitchen, it’s really worth a
try!
In a bowl, crack two or three eggs and
whisk thoroughly to combine the whites and yolks. Don’t add any salt or other
seasonings; do this with just eggs. Transfer to a nonstick pan on a burner set to heat
as low as possible.
Stir continuously with a silicone spatula, doing a “random walk” so that your
spatula hits all parts of the pan. And low heat means really low heat: there’s no need
for the pan to exceed 160°F / 71°C, because enough of the proteins in both the yolks and
whites denature below that temperature and the proteins will weep some of their water as
they get hotter. If your heat source is too hot, pull the pan off the stovetop for a
minute to keep it from overheating. If you see any curds (lumps of scrambled eggs)
forming, your pan is getting too hot.
Stir continuously to avoid hot spots so that the eggs are kept at a
uniform temperature. If you have an IR thermometer, make sure your pan doesn’t
exceed 160°F / 71°C.

Continue stirring until the eggs have set to a custard-like consistency. When I
timed myself, this took about 20 minutes, but you might reach this point in as few as 15
minutes or upward of half an hour.
2.3. Oven-Poached Eggs
Here’s a simple way to cook eggs for a brunch or appetizer. In an individually sized
oven-safe bowl (ideally, one that you can serve in), add:
|
Breakfast version
|
Dinner version
|
|---|
|
1 cup (30g) fresh chopped spinach
|
½ cup (100g) crushed tomatoes
|
|
3 tablespoons (20g) grated mozzarella cheese
|
¼ cup (50g) black beans (canned are
easiest)
|
|
3 tablespoons (40g) heavy cream
|
½ cup (50g) grated mozzarella cheese
|
|
4 teaspoons (20g) butter
| |
Create a “well” in the center of the ingredients by pushing the food into a ring
around the edges of the bowl. Crack two eggs into the well, add a pinch of salt and some
fresh ground pepper, cover with aluminum foil, and bake in a preheated oven set to 350°F
/ 180°C until the egg is set, about 25 minutes. (You can use a probe thermometer set to
beep at 140°F / 60°C.) Try adding some crushed red pepper flakes to the breakfast
version or sriracha sauce to the dinner version.
2.4. Pasteurized Eggs
While salmonella is quite rare in uncooked eggs, with estimates being somewhere
around 1 in 10,000 to 20,000 eggs carrying the bacteria, it does occur in the laying hen
populations of North America. If you’re cracking a few dozen eggs into a bowl for an
omelet brunch at your local hacker house every week, let’s just say that odds are you’ll
eventually crack a bad egg. Luckily, this isn’t a problem if those eggs are properly
cooked and cross-contamination is avoided.
The real risk for
salmonella in eggs is in dishes that use undercooked eggs that are then served to
at-risk populations (e.g., infants, pregnant women, elderly or immunocompromised
people). If you’re making a dish that contains raw or undercooked eggs—Caesar salad,
homemade eggnog, mayonnaise, raw cookie dough—and want to serve that dish somewhere
where there might be at-risk individuals, you can pasteurize the eggs (assuming your
local store doesn’t happen to carry pasteurized eggs, but most don’t). Pasteurized eggs
do taste a little different, and the whites take longer to whip into a foam, so don’t
expect them to be identical to their raw counterparts.
Since salmonella begins to die at a noticeable rate around 136°F / 58°C and the
proteins in eggs don’t begin to denature until above 141°F / 61°C, you can pasteurize
eggs to reduce the quantity of salmonella, should it be present, to an acceptable level
by holding the egg at a temperature between these two points. The FDA requires a
10,000-fold reduction (5 log10 in food safety lingo), which can
be achieved by holding the egg at 141°F / 61°C for 3.5 minutes (according to Margaret
McWilliams’s Foods: Experimental Perspectives, Fifth Edition, from
Pearson Publishing).
2.5. The 60-Minute Slow-Cooked Egg
Going back to our earlier discussion of time and temperature, when food is left in
an environment long enough, its temperature will come to match that of its environment.
Therefore, if we immerse an egg in water held at 145°F / 62.7°C, it follows that the
proteins in the white and the yolk that denature at or below that temperature will
denature and coagulate, and those that denature above that temperature will remain
unaltered.
The added benefit of this method is that the egg cannot
overcook. “Cooking” is effectively the occurrence of chemical reactions in
the food at different temperature points, and holding the egg at 145°F / 62.7°C will not
trigger any reactions that don’t occur until higher temperatures are reached. This is
the fundamental concept of sous vide cooking. For a sous
vide–style cooked egg, immerse an egg in water that is maintained at 145°F / 62.7°C for
one hour. As you’ll see, sous vide cooking has some incredible properties that greatly
simplify the time and temperature rule.
Note:
Your average, run-of-the-mill (or is that run-of-the-yard?) chicken laid only 84
eggs per year a century ago. By the turn of the millennium, improvements in breeding
and feed had pushed this number up to 292 eggs per year—almost 3.5 times more. And,
no, science has not yet figured out which came first.