If all this
talk about collagen and texture isn’t gelling for you, do the following
experiment.
Take a few pieces of beef stew meat, and proceed as though you’re making beef stew.
Once your beef is in the slow cooker, set a timer for 30 minutes.
After 30 minutes, remove a few pieces of the beef. Use a probe thermometer on one to
record the internal temperature; it should register somewhere around 160–180°F /
71–82°C, although it’ll depend on your slow cooker. Stash the 30-minute sample in a
container in your fridge.
After six hours of stewing, repeat the procedure: remove a few pieces, verify that
the temperature is about the same, and stash the second batch in a second container in
the fridge. (You could heat up the 30-minute batch, but then we’d be changing more than
one variable: who’s to say that reheating doesn’t change something?)
Once both samples are cold, do a taste comparison. Got kids? Do a single-blind
experiment to remove the placebo effect: blindfold the kids and don’t let them know
which is which. Got a spouse and kids? Do a double-blind experiment to control for both
placebo effect and observer bias: have your significant other scoop the beef into the
containers and label them only “A” and “B,” not telling you which is which, and then go
ahead and administer the blindfold test to your kids.

4. 158°F / 70°C: Vegetable Starches Break Down
Whereas meat is predominately proteins and fats, plants are
composed primarily of carbohydrates such as cellulose, starch, and pectin. Unlike proteins
in meat, which are extremely sensitive to heat and can quickly turn into shoe leather if
cooked too hot, carbohydrates in plants are generally more forgiving when exposed to
higher temperatures. (This is probably why we have meat thermometers but not vegetable
thermometers.)
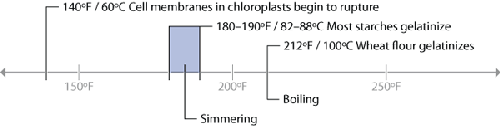
Temperatures related to plants and cooking.
Cooking starchy vegetables such as potatoes causes the starches to gelatinize (i.e.,
swell up and become thicker). In their raw form, starches exist as semicrystalline
structures that your body can only partially digest. Cooking causes them to melt, absorb
water, swell, and convert to a form that can be more easily broken down by your digestive
system.
As with most other reactions in cooking, the point at which starch granules gelatinize
depends on more than just the single variable of temperature. The type of starch, the
length of time at temperature, the amount of moisture in the environment, and processing
conditions all impact the point at which any particular starch granule swells up and
gelatinizes.
Leafy green vegetables also undergo changes when cooked. Most noticeably, they lose
their green color as the membranes around the chloroplasts in the cells rupture. This same
rupturing and damage to the cell structure is what improves the texture of tougher greens
such as Swiss chard and kale.
For starchy plants (think potatoes), cook them so that they reach the temperature at
which they gelatinize, typically in the range of 180–190°F / 92–99°C. For green leafy
plants, sauté the leaves above 140°F / 60°C to break down the plant cell structure.
Note:
Cellulose—a.k.a. fiber—is completely indigestible in its raw form and gelatinizes at
such a high temperature, 608–626°F / 320–330°C, that we can ignore it while discussing
chemical reactions in cooking.
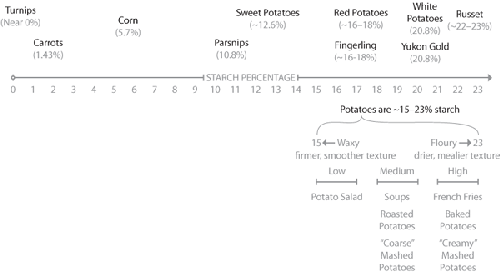
Starch levels in common vegetables.
Microwave ovens make quick work of
cooking veggies. In a microwave-safe container, place asparagus stalks with the bottoms
trimmed or snapped off, and add a thin layer of water to the bottom. Put the lid on, but
leave it partially open so that steam has a place to escape. Microwave for two to four
minutes, checking for doneness partway through and adding more time as necessary. 
Notes This technique cooks the food using two methods: radiant heat
(electromagnetic energy in the form of microwaves) and convection heat (from the
steam generated by heating the water in the container). The steam circulates
around the food, ensuring that any cold spots (areas missed by the microwave
radiation) get hot enough to both cook the food and kill any surface bacteria that
might be present. Try adding lemon juice, olive oil, or butter and sautéed, crushed
garlic to the asparagus.
|
Pectin is a polysaccharide found in
the cell walls of land plants that provides structure to the plant tissue. It breaks
down over time, which is why riper fruits become softer.
Cooking also breaks down pectin, and as a kitchen chemistry experiment, you can
capture the pectin from cooked fruits. It’s an easy way to see that some food additives
aren’t so industrial after all, at least not in their sources.
The pectin we use in cooking—primarily in jams and jellies, as a thickener—is
divided into two broad types: low- and high-methoxyl. High-methoxyl pectin requires a
high concentration of sugar in order to gel; low-methoxyl pectin will gel in the
presence of calcium. (The difference between the two types has to do with the number of
linkages in the molecular structure.)
If you’re making jams or jellies, using a low-methoxyl pectin (such as Pomona’s
Pectin) removes the variable of sugar concentration.
Making your own pectin is similar to making your own gelatin: start with a couple of
pounds of tissue, boil away, and then filter it out. Instead of animal bones, pectin
comes from the “bones” of cell walls in plant tissue.
Start with a few pounds of crisp apples. (The firmer the better! They don’t need to
be ripe.) Chop them into quarters and place the pieces in a stockpot. Cover with water
and simmer on low for several hours, stirring occasionally. (This is exactly the way
stock is made.) After several hours, you should have a slushy sauce. Filter this through
a strainer. The slimy liquid that you filter out is the pectin.
Using homemade pectin will be a bit trickier than Pomona’s Pectin, for two reasons.
First, it’s high-methoxyl pectin, so you’ll need to have a proper balance of sugar in
whatever you’re attempting to gel. And secondly, the concentration of pectin to water
will be unknown, so you will have to experiment some. Add a small quantity and test if
it gels; if not, add more. If the liquid pectin seems too thin, you can boil it down
further to create a more concentrated pectin.