3. 154°F / 68°C: Collagen (Type I) Denatures
An animal’s connective tissues provide structure and
support for the muscles and organs in its body. You can think of most connective
tissues—loose fascia and ligaments between muscles as well as other structures such as
tendons and bones—as a bit like steel reinforcement: they don’t actively contract like
muscle tissue, but they provide structure against which muscles can pull and
contract.
Temperatures related to collagen hydrolysis and the resulting
gelatin.
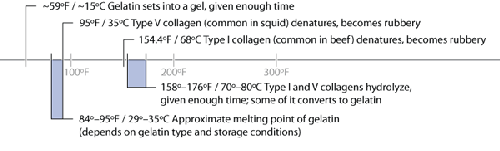
The most common type of protein in connective tissue is collagen, and while there are
several types of collagen in animals, from a culinary perspective, the main chemical
difference between the different types of collagen is the temperature at which they
denature. In cooking, collagen shows up in two different ways: either as discrete chunks
(e.g., tendons, silverskin) outside of the muscle, or as a network that runs through the
muscle. Regardless of its location, collagen is tough (it provides structure, after all)
and becomes palatable only given sufficient time at sufficiently high temperatures.

It’s easy to deal with collagen that shows up as discrete pieces: get rid of it by
cutting it off. For cuts of meats that have a thin layer of connective tissue on them
(called silverskin, presumably because of its somewhat iridescent
appearance), cut off as much as possible and discard it. Beef tenderloin cuts commonly
have a side with this layer; trim off as much as possible before cooking.
Chicken breasts also have a small but noticeable tendon connected to the chicken
tenderloin. Uncooked, it’s a pearlescent white ribbon. After cooking, it turns into that
small white rubber-band-like thing that you can chew on endlessly yet never get any
satisfaction from. Generally, this type of collagen is easy to spot, and if you miss it,
it’s easy to notice while eating and can be left on the plate.
However, for the other kind of collagen found in some cuts of meat—collagen that forms
a 3D network through the muscle tissue—the only way to remove it is to convert it to
gelatin via long, slow cooking methods. Unlike muscle proteins—which in cooking are either
in a native (i.e., as they are in the animal), denatured, or hydrolyzed state—collagen,
once hydrolyzed, can enter
a coagulated (gelled) state. This property opens up an entirely new world of
possibilities, because gelatin gives meats a lubricious, tender quality and provides a
lip-smacking goodness.
In its native form, collagen is like a rope: it’s a linear molecule composed of three
different strands that are twisted together. The three strands are held together by weak
secondary bonds (but there are a lot of them!) and stabilized by a small number of
crosslinks, which are stronger covalent bonds.
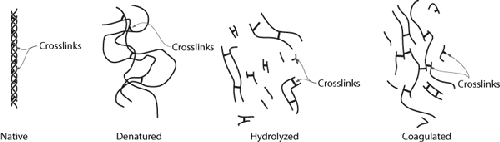
Collagen in its native form is a triple helix, held together in its
helical structure by secondary bonds (left) and stabilized by crosslinks. Under
heat, the secondary bonds break and the protein becomes denatured, but the
crosslinks between the strands continue to hold the structure together (second from
left). Given sufficient heat and time, the strands in the triple helix themselves
break down via hydrolysis (third from left) and, upon cooling, convert to a loose
network of molecules (right) that retains water (a gel).
Note:
Covalent bonds are bonds where the electrons from an atom in
one location are shared with another atom.
In addition to being crosslinked, the strands also form a helical structure because of
secondary bonds between different regions of the same molecules. You can think of it
something like a braided rope, where each strand wraps around the other two strands. It
has a “curl” to it because the internal structure finds its optimal resting place in that
shape.
Under the right conditions—usually, exposure to heat or the right kinds of acids—the
native form of collagen denatures, losing its linear structure and untwisting into a
random mess. With the addition of sufficient heat, the molecules in the structure will
vibrate enough to overcome the electromagnetic energy that caused the structure to twist
up in the first place, leading it to lose its helical structure and denature.
Acids can also denature the collagen protein: their chemical properties provide the
necessary electromagnetic pull to disrupt the secondary bonds of the helical structure.
It’s only the twisting that goes away during denaturing in collagen; the crosslinks remain
in place and the strands remain intact. In this form,
collagen is like rubber—it actually is a rubber from a material science point of view—and
for this reason, you’ll find its texture, well, rubbery.
Given even more heat or acid, though, the collagen structure undergoes
another transformation: the strands themselves get chopped up and
lose their backbone, and at this point the collagen has no real large-scale structure
left. This reaction is called hydrolysis: thermal hydrolysis in the
case of heat, acid hydrolysis in the case of, you guessed it, acid.
It’s possible to break up the collagen chemically, too: lysosomal enzymes will attack
the structure and “break the covalent bonds” in chem-speak, but this isn’t so useful to
know in the kitchen.
Note:
For fun, try marinating a chunk of meat in papaya, which contains an enzyme, papain,
that acts as a meat tenderizer by hydrolyzing collagen.
One piece of information that is critical to understand in the kitchen, however, is
that hydrolysis takes time. The structure has to literally untwist and break up, and due
to the amount of energy needed to break the bonds and the stochastic processes involved,
this reaction takes longer than simply denaturing the protein.
Hydrolyzing collagen not only breaks down the rubbery texture of the denatured
structure, but also converts a portion of it to gelatin. When the collagen hydrolyzes, it
breaks into variously sized pieces, the smaller of which are able to dissolve into the
surrounding liquid, creating gelatin. It’s this gelatin that gives dishes such as braised
ox tail, slow-cooked short ribs, and duck confit their distinctive mouthfeel.
Since these dishes rely on gelatin for providing that wonderful texture, they need to
be made with high-collagen cuts of meat. Trying to make a beef stew with lean cuts will
result in tough, dry meat. The actin proteins will denature (recall that this occurs at
temperatures of 150–163°F / 66–73°C), but the gelatin won’t be present in the muscle
tissue to mask the dryness and toughness brought about by the denatured actin. Don’t try
to “upgrade” your beef stew with a more expensive cut of meat; it won’t work!
“Great,” you might be thinking, “but how does any of this tell me whether I need to
slow-cook a piece of meat?” Think about the piece of meat (or fish or poultry) that you’re
working with and consider what part of the animal it comes from. For a land-based animal,
those regions of the animal that bear weight generally have higher levels of collagen.
This should make sense: because the weight-bearing portions have a higher load, they need
more structure, so
they’ll have more connective tissue. This isn’t a perfect rule of thumb, though, and cuts
of meat generally have more than one muscle group in them.
For animals like fish, which don’t have to support their weight on land, the collagen
levels are much lower. Squid and octopus are notable exceptions to this weight-bearing
rule, because their collagen provides the equivalent support that bone structures do for
fish.
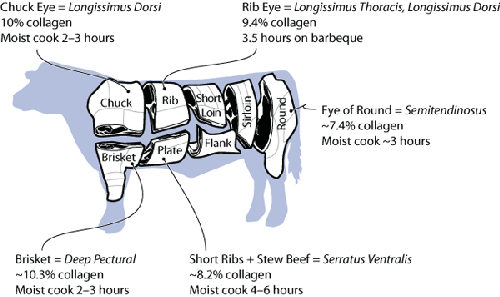
When cooking a piece of meat, if it’s from a part that is responsible
for supporting the animal’s weight (primarily muscles in the chuck, rib, brisket,
and round), it’ll probably be higher in collagen and thus need a longer cooking
time.
Note:
Older animals have higher levels of collagen. As animals age, the collagen structure
has more time to form additional crosslinks between the strands in the collagen helix,
resulting in increased toughness. This is why older chickens, for example, are
traditionally cooked in long, slow roasts. (The French go so far as to use different
words for old versus young chickens: poule instead of
poulet.) Most commercial meat, however, is young at time of
slaughter, so the age of the animal is no longer an important factor.
The other easy rule of thumb for collagen levels is to look at the relative price of
the meat: because high-collagen cuts require more work to cook and come out with a
generally drier texture, people tend to favor other cuts, so the high-collagen cuts are
cheaper.