Your health will suffer if your body is
overly acidic. But you can control it through diet. We show you how.
We in the West have turned a deaf ear to
the wisdom of the ancients: that the most effective antidote to illness is in
nature’s larder. Instead of stocking up on the seasonal, fresh, unrefined foods
that our bodies crave, we gorge on fat, starch, sugar, salt and chemicals when
we become ill.

In
its natural state the pH of the body is slightly alkaline. It must maintain
this for optimal good health
Remedies to cure the effects of bad
nutrition inevitably include some fact, fiction, good sense and
let’s-jump-on-the-bandwagon fads. The acid/alkaline diet sounds like just
another catchy title for a bestseller, but behind the hype there are guidelines
rooted in a simple truth: we are what we eat.
What is the acid/ alkali balance?
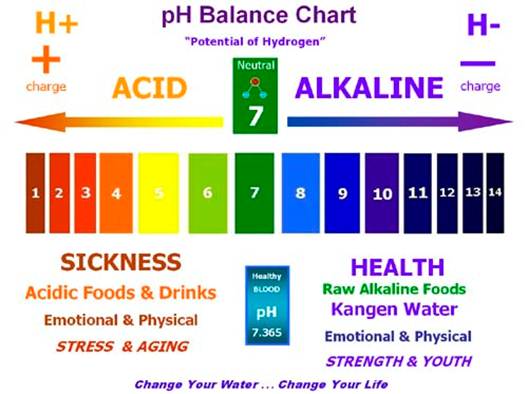
The acid/ alkali balance depends on the
concentrate of hydrogen ions in the body, which in turn determines the pH
(power of hydrogen) of the blood. The scale to measure pH goes from one (most
acid) to 14 (most alkaline). For proper cell function, the pH of blood must be
in the narrow, slightly alkaline range of 7.35-7.45.
The body regulates pH automatically, but
it’s these small imbalances that we refer to when we talk about being acidic.
It would take trauma or an illness, such as kidney failure, to cause a major
fluctuation in pH balance, resulting in a nasty condition known as metabolic
acidosis.
“There are many forms of acidosis,” explains
dietitian Cornelia Owens, from the Nutritional Information Centre at the
University of Stellenbosch (NICUS). “Metabolic acidosis is associated with a
decrease in alkalinity (also called “base”), when there is a drop in the level
of bicarbonate in the fluid outside the veins and arteries. This kind of
disruption is serious. Treatment is essential as the results can range from
electrolyte imbalances to metabolic mayhem and death”
How is pH balance regulated?
“The body has three sophisticated
mechanisms to regulate the pH,” says Owens. “The kidneys do it through hydrogen
secretion and bicarbonate resorption. The lungs regulate it by altering either
the rate or depth of breathing in order to retain carbon dioxide, which is
alkaline. And then there’s a process called buffering, in which alkali reserves
in the cells are used to counter the large amounts of acid produced through the
food we eat and through normal tissue metabolism”
It’s this third area where diet makes a
difference. Regardless of what we eat, the body must maintain the pH balance
for life to be sustained. When food is digested, the residue (also called
“ash”) is either acidic or alkaline. A balanced diet contains the right
proportions of both; if it’s lacking in fruit and vegetables, most of which
have an alkalizing effect, the body will scout around for other sources of
alkali to ensure that the pH remains stable.
Bone has a reliable stock of alkali salts,
but too much plundering of this valuable store will obviously weaken them,
making the person susceptible to osteoporosis and fractures. The American
Journal of Clinical Nutrition reports that a seven-year study conducted at the
University of California on 9 000 women showed that those who have chronic
acidosis are at greater risk for bone loss than those who have normal pH
levels. It found that many of the hip fractures prevalent among middle-aged
women are connected to acidity caused by a diet high in animal foods and low in
vegetables. This is because the body borrows calcium from the bones in order to
balance pH.